This week, we deep dive into a paper recently published in Nature Communications. The study was led by Allison M. Savoie, affiliated with the Coastal Sciences Division of the Pacific Northwest National Laboratory in Sequim (WA, United States).
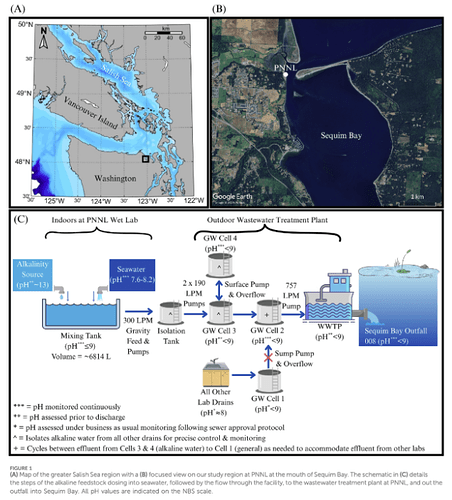
This study reports a small-scale field trial in which electrochemically-derived alkaline seawater was discharged into a coastal outfall in Sequim Bay (Washington State, USA) via a wastewater-treatment plant line. The goal was to test the feasibility of ocean alkalinity enhancement (OAE) for marine CO₂ removal by increasing seawater pH and alkalinity. The experiment showed that pH at the outfall‐adjacent sensors rose from around 7.5–7.7 baseline to peaks near 8.3 during the pulses, while other water quality parameters (temperature, salinity, oxygen, turbidity) remained essentially unchanged. Although the alkalinity signal diluted rapidly (within a few meters), the proof-of-concept of controlled alkaline discharge was shown. The authors conclude this demonstrates the technical viability of a conservative OAE release and provides a basis for up‐scaling further trials.
This study relies on a novel electrochemical generation method (via bipolar membrane electrodialysis) to produce aqueous alkalinity from seawater, which is then discharged back into the ocean, differently from most previous studies focusing on solid alkaline materials (e.g., minerals) or brines. Bringing together industry, academic, and federal partners, this study provides a real-world demonstration of coastal outfall deployment through an integrative “lab-to-sea” setup linking OAE with infrastructure integration and regulatory compliance.
The background seawater pH (NBS scale) in Sequim Bay was measured between 7.5 and 7.7 during the field tests. The mixing tank raised seawater pH up to the permitted maximum (9.0) and maintained it within ±0.2 during discharge. At the outfall, peaks of pH ≈ 8.3 (measured through YSI sensors) and 8.1 (measured through SAMI-pH sensors) were recorded across four discharge pulses. Importantly, the elevated alkalinity and pH did not measurably alter temperature, salinity, turbidity, or dissolved oxygen in the adjacent waters, suggesting limited immediate side-effects in that small-scale deployment. The spatial imprint of the alkaline plume was very local — detectable only within ~2.5 m of the discharge pipe – which underscores both the rapid dilution in coastal settings and the challenge of scale‐efficacy trade-offs. The authors present this pilot as a conservative demonstration: they show that this method works technically and procedurally, yet acknowledge that much larger volumes and longer‐term studies should follow to evaluate CO₂ uptake impacts, ecological responses, and cost‐effectiveness at scale.
Here is a list of the main takeaways of this paper:
- Electrochemically-derived aqueous alkalinity can be safely discharged via a wastewater treatment plant outfall into a coastal bay and monitored in real‐world conditions.
- Peak pH values at the outfall (≈ 8.3) were achieved while other water quality metrics premained unchanged, indicating minimal immediate environmental disruption.
- The effective spatial reach of the alkaline signal was very limited (~2.5 m from discharge), highlighting the challenge of achieving meaningful CO₂ uptake or ocean acidification mitigation at a small scale.
- Infrastructure integration (leveraging a wastewater plant) and permit‐compliant monitoring (using NBS pH scale) are feasible, pointing to realistic deployment pathways for OAE technologies.
- Scaling up (both in volume and duration) is necessary to assess the true potential for durable CO₂ removal and broader marine ecosystem impacts.
Read the full paper here: Novel field trial for ocean alkalinity enhancement using electrochemically derived aqueous alkalinity